
 NKCP, a purified filtrate of Bacillus subtilis var. natto culture is a food based extract of “natto”, a Japanese traditional fermented food made from soybean. Purification to remove most of distinctive odor of natto and its vitamin K2 yields an easy-to-eat food that has a wide variety of uses as a functional food. NKCP contains proteolytic enzymes secreted by Bacillus subtilis var. natto (Bacillus subtilis var. natto-produced protein), which balance clotting mechanisms in the blood.
NKCP, a purified filtrate of Bacillus subtilis var. natto culture is a food based extract of “natto”, a Japanese traditional fermented food made from soybean. Purification to remove most of distinctive odor of natto and its vitamin K2 yields an easy-to-eat food that has a wide variety of uses as a functional food. NKCP contains proteolytic enzymes secreted by Bacillus subtilis var. natto (Bacillus subtilis var. natto-produced protein), which balance clotting mechanisms in the blood.
In vitro and clinical studies have demonstrated that the consistent intake of NKCP over a prolonged period helps to maintain normal circulation. The safety of NKCP has been demonstrated in safety studies.
Patents (Production process for purified filtrate of Bacillus subtilis var. natto culture)
Japan (No.3532503)
The “People's Health Promotion Campaign for the 21st Century (Healthy Japan 21)” was launched in 2000 by the Ministry of Health, Labour and Welfare. The purposes of this campaign are to reduce premature death, prolong optimal health, and improve the quality of life. Simply put, it focuses on longevity accompanied by optimal health. Cardiovascular and cerebrovascular diseases account for about 30% of causes of death in Japan, about 25% in the world. To lower mortality rates while in the prime of life, it is very important to prevent cardiac and cerebrovascular diseases.
A patient with cardiovascular disease may evade a fatal cardiovascular event, but eventually their quality and duration of life will be compromised.
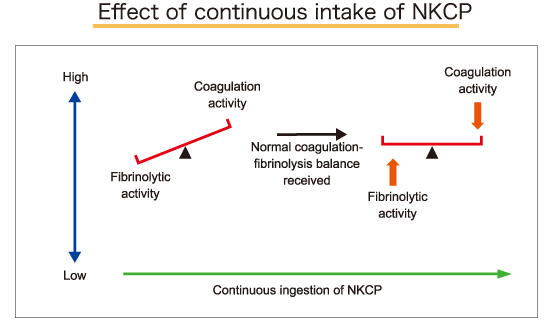
Contemporary lifestyles choices are associated with an increased risk of thrombus formation and subsequent ischemic heart disease and cerebrovascular disease. It has recently been demonstrated that the etiology of travel induced thrombosis, also known as “economy class syndrome”, is Deep Vein Thrombosis (DVT), which may result in a pulmonary embolism. DVT may develop as a result of prolonged sitting in cramped quarters related to both air and auto travel. Additionally, common ailments such as stiff shoulder muscles and leg edema may be caused by insufficient peripheral circulation, caused by excess blood viscosity. The key to preventing DVT and insufficient peripheral circulation is to balance coagulation and fibrinolysis, to prevent thrombus formation and to enhance overall cardiovascular health and longevity. NKCP may be a critical component to achieve such balance.
NKCP was developed based on the traditional Japanese food, natto. Natto contains constituents that enhance the fibrinolysis system in favor of clot lysis. Naturally occurring proteolytic enzymes produced by Bacillus subtilis var. natto dissolve clots in a balanced manner, without causing excessive blood thinning. Recent research has shown that other additional constituents from Bacillus subtilis var. natto produce a substance that acts to inhibit blood coagulation, thereby complementing the known fibrinolytic activity to improve blood viscosity. Based on these insights, it is probable that consistent consumption of natto may lower the overall risk of thrombus formation. However, compliance of such a recommendation may be poor, due to natto's potentially objectionable odor and flavor. Additionally, natto consumption may be contraindicated for patients on anti-coagulant therapy due to its high vitamin K2 content. Furthermore, the proteolytic enzyme content may vary greatly in commercially available natto, resulting in inconsistent anti-coagulant activity.
NKCP was therefore developed to provide a raw material for food products which corrects the drawbacks of natto. NKCP is produced by fermenting the bacillus in a liquid medium containing soybean extract and then partially purifying the peptidase. The odor, bacterial body and vitamin K2 content are reduced to a negligible level. NKCP is designed to contain a constant amount of peptidase.
• NKCP is extracted from Bacillus subtilis var. natto and it is free of the undesirable odor and viscous texture of natto.
• NKCP's purpose is three-fold.
To function as (1) an anticoagulant (2) thrombolytic and (3) decreases blood viscosity.
• The majority of the vitamin K2 has been eliminated, therefore it is less antagonistic to other drugs such as warfarin.
• NKCP is standardized to contain specific levels of proteolytic enzymes secreted by Bacillus subtilis var. natto.
• The principal functional enzyme (protease) is stable at pH 6.0-10.0 and at temperatures 60℃ or below.
• The safety of NKCP has been confirmed in many animal and human studies.
• The production process for this purified filtrate of Bacillus subtilis var. natto culture is registered under Patent No. 3532503 in Japan.
125-500mg/day
NKCP is shown to have the following effects:
a. Inhibiting thrombus formation in vitro and in vivo
b. Decreasing the viscosity of blood in vitro and in vivo
c. Lysing thrombi in vitro and in vivo
The coagulation/fibrinolysis system is comprised of a series of complicated reactions designed to maintain the balance between healthy circulation and prevention of excess bleeding. Many factors can influence this system, however, it is not easily disrupted. In the event that the system shifts towards excess thrombus formation, it is challenging to return the system back to balance. Because it is difficult to lyse a formed thrombus, the emphasis should be placed on prevention of thrombus formation rather than on thrombolysis.
By inhibiting thrombus formation and decreasing blood viscosity, orally administered NKCP helps maintain balance, shifts blood away from clot formation, and enhances circulation throughout the body.
Reduced risk of thrombus formation requires: 1) clot formation prevention 2) maintenance of normal blood viscosity and 3) lysing blood clots (thrombi).
NKCP, derived from Bacillus subtilis var. natto, has been shown to perform these three functions.
(1) Anticoagulant effect
(2) Action of preventing increase in blood viscosity
(3) Thrombolytic effect
| Single-dose | LD50>5,000mg/kg |
| Repeated-dose |
NOAEL Males: > 1,325mg/kg body weight/day Females: > 1,541mg/kg body weight/day |
| Mutagenicity | Negative (± metabolic activation) |
|
Antigenicity (guinea pigs) |
Negative for active systemic anaphylactic reaction (ASA) and passive cutaneous anaphylactic reaction (PCA) |
|
Effect on bleeding time (rats) |
In rats orally given NKCP, a 0.5mm incision was made in the tail tip after 1 hour to measure bleeding time. NKCP at 300mg/kg did not prolong the bleeding time. |
|
Interaction with warfarin (rats) |
NKCP at 250mg/kg was administered into the duodenum by the in situ loop method in rats, in which bleeding time was delayed by treatment with warfarin, and blood collected after 6 hours was measured for coagulation time. The warfarin treatment significantly prolonged the coagulation time in comparison with the control group, but no added delay of coagulation was observed in the warfarin + NKCP treatment group compared with warfarin treatment group. |
|
Long-term administration (humans) |
Twenty-three healthy adults were given NKCP at 250mg/day for 12 weeks, and no clinically significant adverse events were observed. There were no statistically significant changes in hematological or biochemistry tests. |
| Five healthy adults were given NKCP at 750mg/day for 6 consecutive weeks to study and observe changes in laboratory test values (hematological tests, biochemistry tests, and blood coagulation/fibrinolysis parameters) and adverse events. As a result, ELT shortened, t-PA decreased, and thromboplastinogen activity test (TAT) increased but all values were within normal range. In addition, no adverse events were observed, suggesting NKCP safety. | |
|
High dose administration (humans) |
Eight healthy adults were given NKCP at 1,250mg/day for 7 consecutive days. Observation of clinical signs and laboratory tests were utilized to evaluate NKCP safety. There were no clinically significant adverse events. There were no abnormal changes in hematological or biochemistry tests. |
The sample solution is warmed at 37°C with the synthetic chromogenic substrate S-2251 (H-D-valyl-L-leucyl-L-lysine-p-nitroanilide dihydrochloride) as a substrate and the absorbance at 405nm is determined. The enzyme activity is defined as 1 unit when 1nmol of p-nitroaniline per minute is released.
ELISA (enzyme-linked immunosorbent assay) uses rabbit-specific antibodies to the Bacillus subtilis var. natto-produced protein, responsible for the peptidase activity, to measure the amount of antigen reacting with the specific antibodies.